The Newly Discovered Glymphatic System
Understanding the Glymphatic System:
Your Brain’s Hidden Waste Clearance Network and Its Role in Cognitive Health
Introduction
The human brain, despite comprising only 2% of our total body weight, consumes approximately 20% of our daily energy and generates substantial metabolic waste that must be efficiently cleared to maintain optimal cognitive function. For decades, scientists puzzled over how the brain accomplished this critical waste removal process, given that the central nervous system lacks the conventional lymphatic vessels found throughout the rest of the body. The answer came with the groundbreaking discovery of the glymphatic system, a sophisticated waste clearance network that has revolutionized our understanding of brain health and neurodegenerative diseases.
The glymphatic system represents one of the most significant neuroscientific discoveries of the 21st century, fundamentally changing how we approach brain health, sleep medicine, and the prevention of cognitive decline. This remarkable system operates primarily during sleep, utilizing cerebrospinal fluid to flush toxic proteins and metabolic waste from brain tissue through specialized channels formed by glial cells. Recent research has established direct connections between glymphatic dysfunction and the development of Alzheimer’s disease, making this system a critical target for therapeutic interventions and preventive strategies.
Understanding the glymphatic system’s function has profound implications for how we approach cognitive health throughout our lifespan. From the role of quality sleep in brain detoxification to the potential benefits of therapeutic interventions that support glymphatic flow, this knowledge empowers individuals to make informed decisions about their neurological wellbeing. As we delve deeper into this fascinating system, we’ll explore the latest scientific research, examine the connections to neurodegenerative diseases, and discuss evidence-based approaches to supporting optimal glymphatic function.
What Is the Glymphatic System?
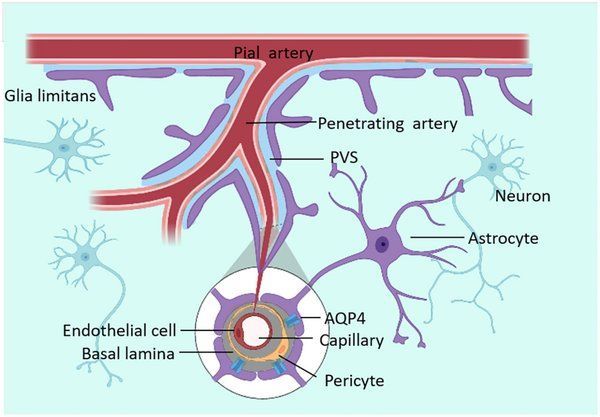
The glymphatic system is a recently discovered macroscopic waste clearance system that utilizes a unique network of perivascular channels formed by astroglial cells to promote efficient elimination of soluble proteins and metabolites from the central nervous system [1]. Unlike the peripheral lymphatic system that drains tissues throughout the body, the glymphatic system is specifically adapted to the unique environment of the brain and spinal cord, where the blood-brain barrier creates a highly regulated microenvironment.
This sophisticated system derives its name from the combination of “glial” cells and “lymphatic” function, reflecting its dependence on glial cells, particularly astrocytes, to create the specialized channels through which cerebrospinal fluid flows. The discovery of this system has resolved a long-standing mystery in neuroscience: how the brain, which lacks conventional lymphatic vessels, manages to clear the substantial amounts of metabolic waste generated by its high metabolic activity.
The glymphatic system operates through a carefully orchestrated process involving multiple brain fluid compartments. The brain consists of four distinct fluid compartments: cerebrospinal fluid (CSF), interstitial fluid, intracellular fluid, and blood vasculature [1]. Cerebrospinal fluid, which comprises approximately 10% of the total fluid volume within the cranial cavity, serves as the primary vehicle for waste removal in this system. The CSF flows through the four ventricles, which are interconnected by channels and foramina, before entering the subarachnoid space surrounding the cortex and spinal cord.
The unique architecture of the glymphatic system centers on perivascular spaces, also known as Virchow-Robin spaces, which are fluid-filled channels that run alongside blood vessels throughout the brain. These spaces are bounded by the blood vessel wall on one side and astrocytic endfeet on the other, creating a specialized conduit for CSF flow. Astrocytes, a type of glial cell, play a crucial role in this system by expressing high levels of aquaporin-4 (AQP4) water channels on their endfeet, which facilitate the rapid movement of water and solutes through brain tissue.
Recent groundbreaking research published in the Proceedings of the National Academy of Sciences has provided the first direct evidence of glymphatic system function in living humans [2]. Using advanced MRI imaging techniques and gadolinium contrast agents, researchers at Oregon Health & Science University demonstrated that cerebrospinal fluid flows into the human brain through distinct perivascular channels, confirming findings previously observed only in animal models. This landmark study involved five volunteers undergoing brain surgery, who received gadolinium injections into their cerebrospinal fluid followed by MRI scans to track fluid movement.
The human study revealed that cerebrospinal fluid doesn’t simply diffuse randomly into brain tissue, as previously thought, but follows specific pathways along blood vessels. As Dr. Juan Piantino, the lead researcher, explained, “This shows that cerebrospinal fluid doesn’t just get into the brain randomly, as if you put a sponge in a bucket of water. It goes through these channels” [2]. This organized flow pattern is essential for efficient waste removal and nutrient distribution throughout the brain.
The Science Behind Glymphatic Function
The glymphatic system operates through a sophisticated mechanism that involves the coordinated interaction of multiple cellular and molecular components. At the heart of this system are astrocytes, star-shaped glial cells that form extensive networks throughout the brain and play crucial roles in maintaining the blood-brain barrier, regulating neurotransmitter levels, and supporting neuronal function. These cells express aquaporin-4 (AQP4) water channels predominantly on their endfeet, which contact blood vessels and create the specialized perivascular spaces essential for glymphatic flow.
The process begins with cerebrospinal fluid production, which occurs primarily at the choroid plexuses located within the brain’s ventricles. The choroid plexuses are highly vascularized structures consisting of specialized epithelial cells that actively transport ions and water from the blood to create CSF. This process involves complex ionic transport mechanisms, particularly the movement of sodium, chloride, and bicarbonate ions, which generate the osmotic gradient necessary for water movement and CSF production [1].
Once produced, cerebrospinal fluid flows through the ventricular system and enters the subarachnoid space surrounding the brain. From there, CSF penetrates the brain parenchyma through perivascular spaces along penetrating arteries. This influx of CSF is driven by arterial pulsations, which create pressure waves that propel fluid into the brain tissue. The pulsatile nature of arterial blood flow serves as a critical driving force for glymphatic circulation, explaining why cardiovascular health is intimately connected to brain waste clearance efficiency.
As cerebrospinal fluid moves through the perivascular spaces, it mixes with interstitial fluid in the brain parenchyma, facilitating the exchange of nutrients and waste products. This mixing process is enhanced by the presence of AQP4 channels on astrocytic endfeet, which allow for rapid water movement and help maintain the pressure gradients necessary for continued flow. The interstitial fluid, now containing metabolic waste products and potentially toxic proteins, is then directed toward venous perivascular spaces for drainage.
The drainage phase of glymphatic function involves the collection of waste-laden interstitial fluid along venous blood vessels, where it eventually reaches the subarachnoid space and drains into the peripheral lymphatic system through various pathways. Recent research has identified several drainage routes, including flow along cranial nerves, particularly the olfactory nerve, and through the cribriform plate of the skull. Additionally, meningeal lymphatic vessels, discovered only in recent years, provide another important pathway for CSF and waste product drainage from the brain.
The efficiency of glymphatic function is significantly influenced by brain state and arousal level. During wakefulness, the extracellular space in the brain is relatively constricted, with an extracellular volume fraction of approximately 14%. However, during sleep, particularly during non-rapid eye movement (NREM) sleep, the extracellular space expands to approximately 22%, creating a 60% increase in the volume available for CSF flow [1]. This dramatic expansion is mediated by changes in astrocyte morphology and the redistribution of AQP4 channels, effectively opening the floodgates for enhanced waste clearance.
The sleep-dependent nature of glymphatic function has profound implications for understanding the biological necessity of sleep. The enhanced waste clearance during sleep may explain why sleep deprivation is associated with cognitive impairment and increased risk of neurodegenerative diseases. During the deep sleep phases, the brain essentially enters a “maintenance mode” where the priority shifts from active information processing to cellular housekeeping and waste removal.
The Sleep-Glymphatic Connection
The relationship between sleep and glymphatic function represents one of the most compelling discoveries in modern neuroscience, providing a mechanistic explanation for why sleep is essential for cognitive health and why chronic sleep deprivation increases the risk of neurodegenerative diseases. The glymphatic system functions most efficiently during sleep, particularly during the non-rapid eye movement (NREM) phases, when the brain undergoes dramatic structural and functional changes that facilitate waste clearance.
During wakefulness, the brain’s extracellular space is relatively compressed, limiting the flow of cerebrospinal fluid through brain tissue. However, as individuals transition into sleep, particularly during deep NREM sleep, astrocytes undergo morphological changes that cause them to shrink, effectively expanding the extracellular space by approximately 60% [1]. This expansion creates wider channels for CSF flow and dramatically increases the efficiency of waste removal from brain tissue.
The mechanism underlying this sleep-dependent enhancement of glymphatic function involves complex changes in astrocyte cell volume and the redistribution of aquaporin-4 (AQP4) water channels. During wakefulness, astrocytes maintain a more expanded morphology that limits extracellular space. The transition to sleep triggers a cascade of cellular events, including changes in potassium and water homeostasis, that cause astrocytes to reduce their cell volume and optimize the positioning of AQP4 channels for maximum water flow.
Noradrenergic signaling plays a crucial role in regulating this sleep-dependent glymphatic enhancement. The neurotransmitter norepinephrine, which is released by neurons in the locus coeruleus, helps maintain astrocyte cell volume during wakefulness. During sleep, noradrenergic activity decreases significantly, allowing astrocytes to shrink and the extracellular space to expand. This mechanism explains why medications that affect noradrenergic signaling can influence glymphatic function and why stress, which increases norepinephrine levels, may impair brain waste clearance.
The timing and quality of sleep are critical factors in glymphatic efficiency. Research has shown that the deepest phases of NREM sleep, characterized by slow-wave activity on electroencephalography, correspond to the periods of maximum glymphatic flow. These slow waves, which represent synchronized neuronal activity across large brain regions, may help coordinate the cellular changes necessary for optimal waste clearance. Disruptions to sleep architecture, such as those seen in sleep disorders or aging, can significantly impair glymphatic function.
Sleep fragmentation and reduced sleep quality have been directly linked to decreased glymphatic clearance efficiency. Studies using animal models have demonstrated that sleep deprivation reduces the clearance of amyloid-beta, a toxic protein associated with Alzheimer’s disease, by approximately 60% compared to normal sleep conditions. This finding has profound implications for understanding how chronic sleep disorders may contribute to the development of neurodegenerative diseases.
The relationship between sleep and glymphatic function also helps explain the cognitive consequences of sleep deprivation. When waste clearance is impaired due to insufficient or poor-quality sleep, toxic metabolites and proteins can accumulate in brain tissue, leading to neuroinflammation, synaptic dysfunction, and cognitive impairment. This accumulation may be particularly problematic for proteins like amyloid-beta and tau, which are implicated in Alzheimer’s disease pathology.
Age-related changes in sleep patterns may contribute to the increased risk of neurodegenerative diseases in older adults. As individuals age, they typically experience reductions in deep sleep duration and quality, which could lead to chronic impairment of glymphatic function and accelerated accumulation of toxic proteins. This connection suggests that interventions aimed at improving sleep quality in older adults may have significant neuroprotective benefits.
The discovery of the sleep-glymphatic connection has important implications for sleep hygiene recommendations and the treatment of sleep disorders. Maintaining consistent sleep schedules, optimizing sleep environment conditions, and addressing underlying sleep disorders may be crucial strategies for supporting long-term brain health. Additionally, this research suggests that the timing of sleep may be as important as its duration, with regular circadian rhythms supporting optimal glymphatic function.
Cholesterol, Apolipoprotein E, and Alzheimer’s Disease
The relationship between cholesterol metabolism, apolipoprotein E (ApoE), and Alzheimer’s disease represents one of the most significant risk factors for neurodegenerative disease development, with direct implications for glymphatic system function and brain waste clearance. Understanding this complex relationship is crucial for appreciating how metabolic dysfunction can contribute to cognitive decline and how supporting glymphatic function may help mitigate these risks.
Apolipoprotein E serves as a major cholesterol carrier in the brain, supporting lipid transport and injury repair throughout the central nervous system [3]. The human APOE gene exists in three polymorphic variants—ε2, ε3, and ε4—which have dramatically different effects on Alzheimer’s disease risk. The ε4 allele represents the strongest genetic risk factor for late-onset Alzheimer’s disease, with individuals carrying one copy of this variant having a 3.2-fold increased risk and those with two copies facing a 14.9-fold increased risk compared to individuals with the more common ε3/ε3 genotype [3].
The brain contains approximately 25% of the body’s total cholesterol despite representing only 2% of total body weight, highlighting the critical importance of cholesterol metabolism for neurological function. Unlike peripheral tissues, the brain synthesizes most of its cholesterol locally due to the blood-brain barrier’s restriction of cholesterol transport from the periphery. This local cholesterol production and metabolism are essential for maintaining neuronal membrane integrity, supporting synaptic function, and facilitating neurotransmitter synthesis.
ApoE4 is associated with significant alterations in brain cholesterol metabolism and lipid homeostasis. Individuals carrying the ε4 allele often exhibit hyperlipidemia and hypercholesterolemia in peripheral tissues, conditions that are associated with increased risk of atherosclerosis, coronary heart disease, and stroke [3]. In the brain, ApoE4 affects the efficiency of cholesterol transport to neurons and influences the clearance of lipid-rich waste products through the glymphatic system.
The connection between ApoE4 and Alzheimer’s disease involves multiple mechanisms, including both amyloid-beta-dependent and independent pathways. ApoE proteins bind to amyloid-beta peptides, influencing their aggregation, clearance, and toxicity. ApoE4 is less efficient at binding and clearing amyloid-beta compared to other ApoE isoforms, leading to increased accumulation of these toxic proteins in brain tissue. This impaired clearance may be partly mediated through reduced glymphatic function, as lipid-rich waste products can accumulate in perivascular spaces and impair CSF flow.
Research has demonstrated that ApoE4 carriers show earlier and more extensive amyloid-beta deposition in the brain, often beginning decades before clinical symptoms of Alzheimer’s disease appear. The frequency of Alzheimer’s disease and mean age at clinical onset vary dramatically based on ApoE genotype: 91% frequency and 68 years of age in ε4 homozygotes, 47% frequency and 76 years of age in ε4 heterozygotes, and 20% frequency and 84 years in ε4 noncarriers [3]. This gene dose-dependent effect underscores the powerful influence of ApoE on disease risk and progression.
The relationship between cholesterol metabolism and glymphatic function extends beyond amyloid-beta clearance. Cholesterol and other lipids must be efficiently transported and cleared from brain tissue to maintain cellular homeostasis. When glymphatic function is impaired, whether due to aging, sleep disorders, or other factors, lipid-rich waste products can accumulate and contribute to neuroinflammation and cellular dysfunction. This accumulation may be particularly problematic for ApoE4 carriers, who already have compromised lipid metabolism and clearance mechanisms.
Emerging research suggests that interventions aimed at supporting glymphatic function may be particularly beneficial for individuals at high genetic risk for Alzheimer’s disease. Strategies that enhance CSF flow and waste clearance, such as optimizing sleep quality, maintaining cardiovascular health, and potentially using targeted therapies, may help compensate for the genetic disadvantages associated with ApoE4 carriage.
The discovery of meningeal lymphatic vessels and their role in brain waste clearance has added another layer of complexity to understanding cholesterol and protein clearance from the brain. These vessels, which drain CSF and associated waste products to peripheral lymph nodes, may be particularly important for clearing lipid-rich particles and could be compromised in individuals with ApoE4-related metabolic dysfunction.
Understanding the interplay between genetic risk factors, cholesterol metabolism, and glymphatic function provides a framework for developing personalized approaches to Alzheimer’s disease prevention. Individuals with known ApoE4 carriage may benefit from more aggressive interventions to support brain waste clearance, including optimized sleep hygiene, cardiovascular risk management, and potentially targeted nutritional or pharmacological interventions that support glymphatic function.
Glymphatic Dysfunction and Neurological Diseases
Impairment of glymphatic system function has emerged as a common pathway in the development and progression of numerous neurological and neurodegenerative diseases, fundamentally altering our understanding of disease mechanisms and therapeutic targets. The accumulation of toxic proteins and metabolic waste products due to compromised glymphatic clearance contributes to neuroinflammation, synaptic dysfunction, and ultimately neuronal death across a spectrum of conditions.
Alzheimer’s disease represents the most extensively studied example of glymphatic dysfunction in neurodegeneration. The hallmark pathological features of Alzheimer’s disease—amyloid-beta plaques and tau neurofibrillary tangles—are both proteins that require efficient clearance through the glymphatic system. When this clearance mechanism is impaired, these toxic proteins accumulate in brain tissue, triggering inflammatory cascades and synaptic damage that ultimately lead to cognitive decline and dementia.
Research has demonstrated that glymphatic function declines significantly with aging, which may explain the increased prevalence of neurodegenerative diseases in older adults. Age-related changes include reduced CSF production, decreased arterial pulsatility that drives glymphatic flow, alterations in astrocyte morphology and AQP4 distribution, and increased perivascular inflammation that can obstruct drainage pathways. These changes create a perfect storm for the accumulation of toxic proteins and metabolic waste products.
Traumatic brain injury represents another condition where glymphatic dysfunction plays a critical role in both acute injury responses and long-term complications. Following brain trauma, the glymphatic system can be severely disrupted due to damage to blood vessels, astrocytes, and the blood-brain barrier. This disruption impairs the clearance of inflammatory mediators, cellular debris, and toxic proteins, contributing to secondary injury mechanisms and increasing the risk of long-term neurodegenerative changes.
Studies have shown that traumatic brain injury can lead to persistent glymphatic dysfunction that may last for months or years after the initial injury. This prolonged impairment may explain why individuals with a history of brain trauma have increased risk of developing Alzheimer’s disease, chronic traumatic encephalopathy, and other neurodegenerative conditions later in life. The accumulation of tau protein, in particular, appears to be accelerated when glymphatic clearance is compromised following brain injury.
Stroke and cerebrovascular disease also significantly impact glymphatic function through multiple mechanisms. Acute stroke can directly damage the vascular structures that support glymphatic flow, while chronic cerebrovascular disease can lead to gradual impairment of the arterial pulsatility that drives CSF circulation. Additionally, the inflammation and blood-brain barrier disruption associated with stroke can further compromise waste clearance mechanisms.
The relationship between cardiovascular health and glymphatic function extends beyond acute vascular events. Conditions such as hypertension, diabetes, and atherosclerosis can all impair the arterial pulsatility and vascular health necessary for optimal glymphatic flow. This connection helps explain why cardiovascular risk factors are also risk factors for dementia and cognitive decline, providing a mechanistic link between heart health and brain health.
Sleep disorders represent a particularly important category of conditions that can severely impair glymphatic function. Sleep apnea, insomnia, and other sleep disturbances can reduce the deep sleep phases when glymphatic clearance is most active, leading to chronic accumulation of toxic proteins and metabolic waste. This relationship may explain why sleep disorders are associated with increased risk of Alzheimer’s disease and other neurodegenerative conditions.
Parkinson’s disease and other synucleinopathies also involve glymphatic dysfunction, particularly in the clearance of alpha-synuclein protein aggregates. The accumulation of alpha-synuclein in Lewy bodies, a hallmark of Parkinson’s disease, may be partly due to impaired glymphatic clearance. Research has shown that enhancing glymphatic function can improve the clearance of alpha-synuclein and reduce associated pathology in animal models.
Multiple sclerosis and other neuroinflammatory conditions can both cause and result from glymphatic dysfunction. Inflammation in the central nervous system can impair astrocyte function and disrupt the cellular mechanisms necessary for efficient waste clearance. Conversely, the accumulation of inflammatory mediators due to impaired glymphatic function can perpetuate and amplify neuroinflammatory processes.
The recognition of glymphatic dysfunction as a common pathway in neurological disease has important implications for therapeutic development. Interventions that enhance glymphatic function may have broad neuroprotective effects across multiple conditions. This has led to increased interest in developing pharmacological agents, medical devices, and lifestyle interventions specifically designed to support brain waste clearance.
Understanding glymphatic dysfunction also provides new perspectives on disease prevention and early intervention. Since glymphatic impairment often precedes clinical symptoms by years or decades, strategies to maintain or enhance glymphatic function throughout life may be crucial for preventing neurodegenerative diseases. This preventive approach represents a paradigm shift from treating established disease to maintaining brain health across the lifespan.
Evidence-Based Approaches to Supporting Glymphatic Function
The growing understanding of glymphatic system function has led to the identification of several evidence-based strategies for supporting brain waste clearance and potentially reducing the risk of neurodegenerative diseases. These approaches range from lifestyle modifications to emerging therapeutic interventions, each targeting different aspects of the complex mechanisms underlying glymphatic function.
Sleep optimization represents the most well-established and accessible intervention for supporting glymphatic function. Given that glymphatic clearance is most active during deep NREM sleep, maintaining consistent sleep schedules, achieving adequate sleep duration, and optimizing sleep quality are fundamental strategies for brain health. Research suggests that adults should aim for 7-9 hours of sleep per night, with particular attention to maintaining regular bedtimes and wake times to support circadian rhythm regulation.
Sleep hygiene practices that specifically support glymphatic function include creating a cool, dark, and quiet sleep environment, avoiding caffeine and alcohol before bedtime, and limiting screen exposure in the evening hours. The sleep position may also influence glymphatic clearance, with some research suggesting that lateral sleeping positions may enhance CSF flow compared to supine or prone positions, though more research is needed to establish definitive recommendations.
Cardiovascular health maintenance is another crucial factor in supporting glymphatic function, as the arterial pulsatility that drives CSF flow depends on healthy cardiovascular function. Regular aerobic exercise has been shown to improve cardiovascular health and may enhance glymphatic clearance through multiple mechanisms, including improved arterial compliance, enhanced sleep quality, and reduced inflammation. Studies suggest that moderate-intensity exercise for at least 150 minutes per week can provide significant cardiovascular and potentially neurological benefits.
Blood pressure management is particularly important for glymphatic function, as both hypertension and hypotension can impair the arterial pulsatility necessary for optimal CSF flow. Maintaining blood pressure within recommended ranges through lifestyle modifications and, when necessary, appropriate medications can help preserve the vascular health essential for glymphatic function. Regular monitoring and management of other cardiovascular risk factors, including diabetes and hyperlipidemia, are also important for long-term brain health.
Hydration status may influence glymphatic function, though the optimal hydration strategies for brain health are still being investigated. Adequate hydration is necessary for CSF production and flow, but excessive fluid intake, particularly before bedtime, can disrupt sleep quality and potentially impair glymphatic clearance. Current recommendations suggest maintaining consistent hydration throughout the day while avoiding excessive fluid intake in the evening hours.
Stress management and mental health support may also play important roles in glymphatic function. Chronic stress can elevate cortisol and norepinephrine levels, which may impair the cellular mechanisms necessary for optimal waste clearance. Stress reduction techniques such as meditation, yoga, deep breathing exercises, and regular relaxation practices may help support glymphatic function by promoting better sleep quality and reducing inflammatory stress responses.
Nutritional factors are increasingly recognized as important modulators of glymphatic function. Omega-3 fatty acids, particularly DHA (docosahexaenoic acid), are essential for maintaining neuronal membrane health and may support glymphatic clearance mechanisms. Antioxidant-rich foods, including berries, leafy greens, and other colorful fruits and vegetables, may help reduce oxidative stress and inflammation that can impair glymphatic function.
Intermittent fasting and caloric restriction have shown promise in animal studies for enhancing glymphatic clearance, possibly through mechanisms involving autophagy and cellular stress responses. However, more research is needed to establish safe and effective fasting protocols for humans, and such approaches should be undertaken with medical supervision, particularly for individuals with underlying health conditions.
Emerging therapeutic approaches for enhancing glymphatic function include pharmacological interventions targeting specific molecular pathways. Research is investigating compounds that can modulate astrocyte function, enhance AQP4 expression and localization, or improve CSF flow dynamics. Some existing medications, such as certain anesthetics and sleep aids, have been shown to influence glymphatic function, though their long-term effects and optimal use for brain health are still being studied.
Non-invasive brain stimulation techniques, including transcranial magnetic stimulation and focused ultrasound, are being investigated for their potential to enhance glymphatic clearance. These approaches may work by modulating neuronal activity, improving sleep quality, or directly influencing CSF flow dynamics. While promising, these techniques are still largely experimental and require further research to establish their safety and efficacy.
The timing of interventions may be crucial for maximizing their benefits for glymphatic function. Some research suggests that interventions implemented during midlife, before significant neurodegeneration has occurred, may be most effective for preventing age-related cognitive decline. This highlights the importance of adopting brain-healthy lifestyle practices early and maintaining them throughout life.
Personalized approaches to supporting glymphatic function may become increasingly important as our understanding of individual risk factors and genetic influences grows. Individuals with genetic risk factors such as ApoE4 carriage may benefit from more intensive interventions, while those with specific sleep disorders or cardiovascular conditions may require targeted therapeutic approaches.
Conclusion and Future Directions
The discovery and characterization of the glymphatic system represents a paradigm shift in our understanding of brain health, providing crucial insights into the mechanisms underlying cognitive aging and neurodegenerative diseases. This remarkable waste clearance system, operating primarily during sleep through specialized channels formed by glial cells, has emerged as a critical target for therapeutic interventions aimed at maintaining cognitive function and preventing neurodegeneration throughout the lifespan.
The evidence linking glymphatic dysfunction to Alzheimer’s disease, traumatic brain injury, stroke, and other neurological conditions underscores the fundamental importance of brain waste clearance for neurological health. The strong connections between sleep quality, cardiovascular health, and glymphatic function provide clear pathways for evidence-based interventions that individuals can implement to support their long-term cognitive wellbeing.
As our understanding of the glymphatic system continues to evolve, several key areas warrant continued research and development. Advanced imaging techniques that can non-invasively assess glymphatic function in living humans will be crucial for developing personalized approaches to brain health and monitoring the effectiveness of therapeutic interventions. The recent success in visualizing glymphatic flow in human subjects represents an important step toward these goals.
The development of pharmacological interventions specifically designed to enhance glymphatic function represents an exciting frontier in neuroscience and drug development. Compounds that can optimize astrocyte function, enhance aquaporin-4 expression, or improve cerebrospinal fluid dynamics may provide powerful tools for preventing and treating neurodegenerative diseases. However, such interventions must be carefully developed and tested to ensure safety and efficacy.
The integration of glymphatic health considerations into clinical practice will require the development of standardized assessment tools and treatment protocols. Healthcare providers will need education and training on the importance of sleep quality, cardiovascular health, and other factors that influence glymphatic function. This integration may lead to new approaches to preventive medicine that prioritize brain waste clearance as a key component of healthy aging.
While emerging therapeutic approaches such as facial massage and lymphatic drainage techniques may provide complementary benefits for overall wellness and stress reduction, their specific effects on brain glymphatic function require further scientific investigation. The most robust evidence currently supports lifestyle interventions focused on sleep optimization, cardiovascular health maintenance, stress management, and overall healthy aging practices.
The personalization of glymphatic health strategies based on individual risk factors, genetic profiles, and health status represents an important direction for future research and clinical application. Individuals with genetic risk factors such as ApoE4 carriage may benefit from more intensive interventions, while those with specific medical conditions may require targeted therapeutic approaches.
The implications of glymphatic research extend beyond individual health to public health policy and healthcare system planning. Understanding the importance of sleep, cardiovascular health, and brain waste clearance for cognitive aging may inform recommendations for work schedules, urban planning, and healthcare resource allocation. The potential for preventing neurodegenerative diseases through glymphatic health maintenance could have profound impacts on healthcare costs and quality of life for aging populations.
As we continue to unravel the complexities of the glymphatic system, it becomes increasingly clear that brain health is intimately connected to overall physiological health and lifestyle factors. The most effective approaches to supporting glymphatic function and preventing cognitive decline will likely involve comprehensive strategies that address sleep, cardiovascular health, stress management, and other modifiable risk factors throughout the lifespan.
The future of glymphatic research holds promise for revolutionary advances in our ability to prevent and treat neurodegenerative diseases. By understanding and supporting the brain’s natural waste clearance mechanisms, we may be able to maintain cognitive function and quality of life well into advanced age, fundamentally changing the trajectory of human aging and neurological health.
The dedicated professionals at Your Body Revolution share a deep passion for lymphatic health and wellness. Our mission is to educate and support our community in understanding the vital importance of proper lymphatic flow for optimal health. We believe everyone deserves access to the knowledge and therapeutic treatments that can enhance their quality of life. Begin your wellness journey with us today by booking a personalized Lymphatic Drainage or Glymphatic Drainage session using the convenient link below.
References
[1] Jessen, N. A., Munk, A. S. F., Lundgaard, I., & Nedergaard, M. (2015). The glymphatic system: A beginner’s guide. Neurochemical Research, 40(12), 2583-2599. https://pmc.ncbi.nlm.nih.gov/articles/PMC4636982/
[2] National Institutes of Health. (2024, October 22). Brain waste-clearance system shown in people for first time. NIH Research Matters. https://www.nih.gov/news-events/nih-research-matters/brain-waste-clearance-system-shown-people-first-time
[3] Liu, C. C., Kanekiyo, T., Xu, H., & Bu, G. (2013). Apolipoprotein E and Alzheimer disease: Risk, mechanisms, and therapy. Nature Reviews Neurology, 9(2), 106-118. https://pmc.ncbi.nlm.nih.gov/articles/PMC3726719/
[4] Marxen, T., Shauly, O., Goel, P., Tsan, T., Faria, R., & Gould, D. J. (2023). The utility of lymphatic massage in cosmetic procedures. Aesthetic Surgery Journal Open Forum, 5, ojad023. https://pmc.ncbi.nlm.nih.gov/articles/PMC10045879/
Disclaimer: This article is for educational purposes only and should not be considered medical advice. Individuals with health concerns should consult with qualified healthcare professionals for personalized medical guidance. The information presented here is based on current scientific research and understanding, which continues to evolve as new discoveries are made.